
Tilo Winkler, Ph.D.
|
Investigator, Associate Professor, Ext Fund Anesthesia, Critical Care and Pain Medicine, Mass General Research Institute |
|
Associate Professor of Anaesthesia Harvard Medical School |
Research Interests
Research Narrative

* Slide 2-3: Imaging Can Identify Early Lung Disease in People with HIV -- ADVANCES IN MOTION - Kohli P et al. J Nucl Med. 2020;https://doi.org/gcf6
Pulmonary imaging and biomedical engineering have led to scientific breakthroughs in the understanding, diagnosis, and treatment of lung diseases. However, the causes for the emergence of heterogeneity in lung disease, its progression, and responses to treatment remain largely unknown. We use advanced imaging methods and computational modeling for the quantitative assessment of ventilation, perfusion, strain, inflammation, blood clotting, and other parameters characterizing disease-specific deviations from healthy conditions. The ultimate goal of our studies is a deeper understanding of the diseases to develop novel treatments and improve patient outcomes.
Positron-Emission Tomography (PET) and Computed Tomography (CT)
Our interdisciplinary team of physicians and engineers has developed advanced imaging methods and modalities that allow us to study a wide range of respiratory diseases and conditions in humans and large animals. Combining PET and CT imaging allows for both structural assessments using the high spatial resolution of CT and functional assessments using the excellent sensitivity of PET scanners for tracers and their kinetics.
Using our imaging methods, we demonstrated for example:
- the emergence of ventilation defects, areas of hypoventilation, during bronchoconstriction
- the predominant location of ventilation defects during bronchoconstriction in the dependent regions of the lungs
- regional hypoperfusion within ventilation defects compared to areas outside
- the failure of hypoxic and hypercapnic vasoconstriction to fully explain the hypoperfusion within ventilation defects, which suggests that the hyperinflation within ventilation defects and the distortion of blood vessels on the outside of the constricting airways may contribute to the regional hypoperfusion
- changes in perfusion in people with HIV and smokers that can identify early lung disease
- distinguished perfusion patterns in exercise pulmonary arterial hypertension at rest
- spatial heterogeneity in perfusion as a vascular biomarker in COPD
- heterogeneity in strain and lung aeration as important factors in early ventilator-induced lung injury
- high metabolic activity as a biomarker of eosinophilic inflammation and airway response in asthma
Computational Modeling
Our team has developed a unique model of airway behavior during bronchoconstriction that could explain the complex behavior of airways that occurs beyond a tipping point in airway narrowing with a positive feedback mechanism leading to the emergence of ventilation defects. This model could for the first time explain the ventilation defects we had found in our imaging studies. Additionally, the quantification of tracer kinetics in our PET imaging studies is based on parameter identification methods and model selection algorithms that are essential for the quality of our quantitative analysis. For both 13NN and 18F-FDG, we developed several tracer kinetic models that capture different characteristics of the lungs, and we implemented advanced parameter identification methods for the integration of these models in our custom imaging analysis software.
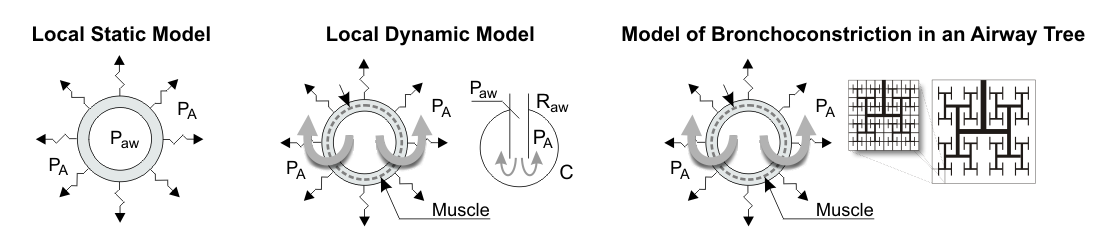
Figure: Comparison of three generations of airway tree models that have been used in asthma research. In contrast to single airway models with local static or local dynamic behavior, the model of bronchoconstriction in an airway tree is a distributed model where airway narrowing beyond a tipping point results in the emergence of clustered severe constriction, which causes ventilation defects (VDefs) - Ref.: Tilo Winkler T. In silico modeling of airway mechanics. Drug Discovery Today: Disease Models. 2007;4(3):125–129. doi: 10.1016/j.ddmod.2007.12.002
The inhaled carbon monoxide (CO) administration in acute lung injury and sepsis-induced ARDS are novel therapeutic approaches currently investigated that require predictions of the CO levels based on a model of CO uptake and elimination in the lungs. Using the uptake kinetics, we have developed a novel method for estimating the endogenous carbon monoxide production and the diffusing capacity of the lung for carbon monoxide.
Summary
The long-term goal of our research is to transform pulmonary imaging through innovations and gain deeper insights into lung diseases and injury. Additionally, our quantitative approaches in imaging and bioengineering aim to advance knowledge in respiratory physiology and biomedical engineering.
Collaborations
The close collaboration between physicians and engineers at the Pulmonary Imaging and Bioengineering Laboratories has been very important for our imaging, computational modeling, and bioengineering projects. Current and past collaborations include MIT, Boston University, Brigham and Women's Hospital, the New England Complex Systems Institute, and several other institutions. Additionally, these collaborations include a very diverse group of international fellows.

Publications
Please visit the most suitable bibliography:
PubMed > including the most recent papers
Harvard Catalyst > with automatic updates and manually added items, e.g., book chapters
Google Scholar > with citation statistics
ORCID >

Profiles:
Harvard Catalyst > including links to co-authors and collaborators at Harvard
ResearchGate >
Harvard Dataverse > a repository including shared data, reports, and code
Websites:
Investigator Profiles > at the Department of Anesthesia, Critical Care and Pain Medicine -- a hub of all investigators
Research in the Department of Anesthesia, Critical Care and Pain Medicine >
| twinkler@mgh.harvard.edu |
| 6177244083 |
|
Edwards Research 60 Blossom Street 410G Boston, MA 02114 |
